As Seen On TV
As Featured on "The Richard & Judy Show"
Lights, Cameras, Action... As the set went quiet and the feature opened with the three subjects attending their preliminary eye examinations at Optical Express in the Strand earlier in the week, to have their sight and cataracts checked by another ophthalmic specialist before starting their six months Ethos Bright Eyes trial, my heart missed a beat as I knew I'd be on live TV any minute. They were each looked at individually in turn and, after their examinations, the ophthalmologist confirmed that all three had cataracts of varying degrees of severity.
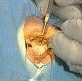
I was sat on the couch beside John Bolger, one of the UK's leading BUPA cataract surgeons and world authority on cataracts operations, and we watched as they rolled a greatly speeded up clip of John performing a cataracts operation while he narrated, quickly explaining what exactly was involved in the operation, and the different steps he was doing as he completed them.
This part was definitely not for the squeamish or faint-hearted among the 2.1 million people viewing the Show on Wednesday evening. I had never seen one before myself, and can now quite understand why Helen Aslam from Essex, one of the two ladies out of the three trial subjects, was so traumatised by her first cataracts operation that she refused to go in for surgery again to have her other eye done, and jumped at the opportunity when her sister saw on the C4 web site that they were looking for people to try our revolutionary new alternative treatment.
We then discussed the Bright Eyes Treatment and benefits with Richard and Judy and I think everyone was a little taken aback by how open John was to the new possibilities, and he even commented at the end that he could possibly see it replacing cataracts operations one day in the future but he would wait to see the results of the trial before commenting on it any further.
So a brilliant feature on National Television and, as you can imagine, the calls have been flooding into our offices with people wanting to know more about Ethos Bright Eyes.
We would like to personally thank again Richard and Judy for inviting us onto their show and to Patrick Ruddy for his tireless efforts in laying down all the groundwork and for organising everything so professionally. And to all the other people at Cactus TV who helped to make it such a wonderful day for us.

We would also like to sincerely thank John Bolger for being open-minded and for all his help in researching Bright Eyes so that we could initiate the trial in the first place - Thank you John!
Follow this link for update after six weeks - Six Week Update
Bright Eyes Products Ingredients:
Antioxidants: N-Acetyl-Carnosine (NAC) - 1.0%
Glycerin (lubricant) - 1.0%
Carboxymethylcellulose sodium (lubricant) - 0.3%
Buffered with borates and potassium bicarbonate and as a preservative
Sterile water (ophthalmic grade isotonic solution, pH 6.3 to 6.5)
Purified benzyl alcohol
Other Information:
Bright Eyes is 100% hypoallergenic
Bright Eyes is 100% free of animal ingredients and may be freely used by vegetarians
Bright Eyes is 100% safe and suitable for use on pets and other animals
Bright Eyes Recommended Usage:
The suggested use of Bright Eyes NAC Eye Drops is to apply 1drop in each eye 5-7 times every day. Those with any kind of eye problem may want to apply 1 to 2 drops several times a day.
Each 5ml bottle should last 30 days at two drops per day or 15 days at four drops per day. A box contains 2 x 5ml bottles; therefore each package can last between 30 and 60 days which represents excellent value for money.
Unopened bottles should be stored in the dark in the refrigerator.
It is also recommended that you take 1 teaspoon of Ethos élan vital supplement in powder form daily.
From our investigations, we have reported that eye drops containing 20 mM carnosine were used to treat 96 patients aged 60 years old having senile cataract of various degrees of maturity, with the duration of the disease from 2 to 21 years. The method is that after stopping all other anti-cataract drugs, patients instilled 1-2 drops of the carnosine containing solution in each eye 3-4 times each day for a period of treatment ranging from 3-6 months. The level of eyesight improvement and the change of lens transparency were considered as an evaluation index of the curative effect of carnosine.
The result shows that carnosine gives a profound effect on primary senile cataract, the effective rate being 100%.
For mature senile cataract, the effect rate is 80%, and positive effects were observed with other types of cataract.
It is significant that no side effect has been found in the observed cases. During recent years, we have also applied carnosine drops containing the same content to nearly 1,000 patients with senile cataract. Our research findings (ready to be published) show similar results.
In addition, we applied carnosine drops to patients aged 48-60 years with various degrees of eyesight impairment but without symptoms of cataract. The course of treatment is from 2 to 6 months. The results demonstrate that carnosine appears to alleviate eye tiredness and comparatively improve eyesight (obviously improve eyesight, giving more clear vision). Subjects reported that carnosine could brighten and relax their eyes.
It is an important point that all the above research on the medical application of carnosine has statistical significance.

Efficacy of N-acetylcarnosine in the treatment of cataracts.
Babizhayev MA, Deyev AI, Yermakova VN, Semiletov YA, Davydova NG, Doroshenko VS, Zhukotskii AV, Goldman IM.
PURPOSE: To evaluate the effects of 1% N-acetylcarnosine (NAC) solution on lens clarity over 6 and 24 months in patients with cataracts.
TRIAL DESIGN: Randomised, placebo-controlled study.
PARTICIPANTS: 49 subjects (76 affected eyes) with an average age of 65.3 +/- 7.0 years with a diagnosis of senile cataract with minimum to advanced opacification in various lens layers. METHODS: 26 patients (41 eyes) were allocated to topical NAC 1% eyedrops twice daily.
The control group consisted of 13 patients (21 eyes) who received placebo eyedrops and 10 patients (14 eyes) who did not receive eyedrops.
MAIN OUTCOME MEASURES: All patients were evaluated at entry and followed up every 2 months for a 6-month period (trial 1), or at 6-month intervals for a 2-year period (trial 2), for best-corrected visual acuity and glare testing. In addition, cataract was measured using stereocinematographic slit-images and retro-illumination examination of the lens. Digital analysis of lens images displayed light scattering and absorbing centres in two- and three-dimensional scales.
RESULTS: The overall intra-reader reproducibility of cataract measurements (image analysis) was 0.830, and glare testing 0.998. After 6 months, 90% of NAC-treated eyes showed improvement in best corrected visual acuity (7 to 100%) and 88.9% showed a 27 to 100% improvement in glare sensitivity.
Topographic studies indicated fewer areas of posterior subcapsular lens opacity and 41.5% of treated eyes had improvement in image analysis characteristics. The overall ratios of image analysis characteristics at 6 months compared with baseline measures were 1.04 and 0.86 for the control and NAC-treated group, respectively (p < 0.001). The apparent benefits of treatment were sustained after 24 months' treatment.
No treated eyes demonstrated worsening of vision. The overall visual outcome in the control group showed significant worsening after 24 months in comparison with both baseline and the 6-month follow-up examination. The overall clinical results observed in the NAC-treated group by the 24-month period of examination differed significantly (p < 0.001) from the control group in the eyes with cortical, posterior subcapsular, nuclear or combined lens opacities. Tolerability of NAC eyedrops was good in almost all patients, with no reports of ocular or systemic adverse effects.
CONCLUSION: Topical NAC shows potential for the treatment and prevention of cataracts.
Publication Types:
Clinical Trial
Randomised Controlled Trial
The photographs below show images of a lens with senile cataract (corticonuclear opacities, grade 4, age 75 years, female), and the subsequent slit images in optical section documenting in the focal plane: (a) marked light scattering in the nucleus and posterior cortical region outlined by the lens optical scanning with the focal plane movement inside the lens thickness; (b) light scattering in the anterior subcapsular, anterior cortical and nuclear regions of the lens; retro-illumination lens images with a focal plane positioned (c) onto the iris and (d) on the posterior lens layers. Opacities in the cortical layers are demonstrated as the white background inclusions in the boundary of the pupil locally masked by the flashlight article. (e) the neutral density step reference wedge captured in the plane of the camera focus and allowing correction for variations in film development and flashlight output.

In the NAC-treated group, 6-month follow-up showed an improvement in VA (7-100%) in 37 of the 41 treated eyes and a significant improvement in glare sensitivity at red and green targets (27-100%) was documented in 16 of the 18 eyes tested. A significant improvement in lens clarity was found in 17 of the 41 eyes as documented by a significant decrease of M and H characteristics during image grading. The NAC-treated eyes had statistically significant differences in VA, glare sensitivity and characteristics of image analysis compared with the control group (p < 0.001) at this time point, as supported by the overall t-test results of the ratio of the follow-up data to the baseline values.
WARNING: Bright Eyes for cataracts was the actual product featured in the Richard & Judy TV Trials and the UK Daily Mail newspaper features. Many other eye drop suppliers are now trying to cash in on the phenomenal success of our Bright Eyes drops and are exploiting our features, testimonials and press coverage saying that they relate to their products which is blatantly untrue! Purchasing these less expensive copy-cat products may lead you to very disappointing results...

